Boost sales with PrestaShop Live Chat & Chatbots
Smartsupp helps thousands of businesses on PrestaShop sell more, save time and be online 24/7 using a combination of chatbots and live chat.
20,000+
Instalations
4.7 ⭐️
on PresaShop marketplace
100,000+
customers all over globe










Enhance your business with the PrestaShop live chat & chatbots
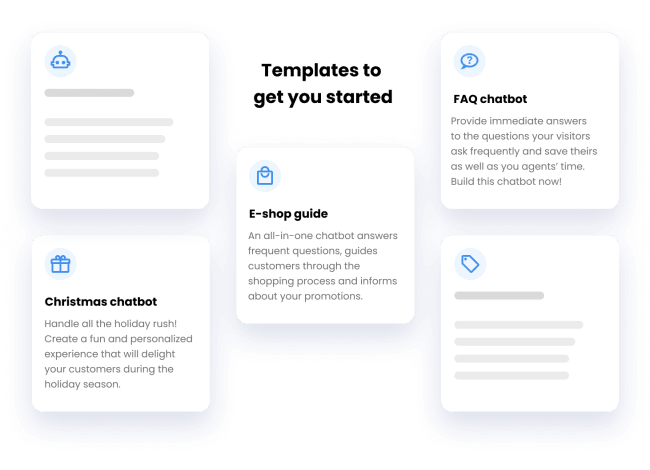
Install in 1 click
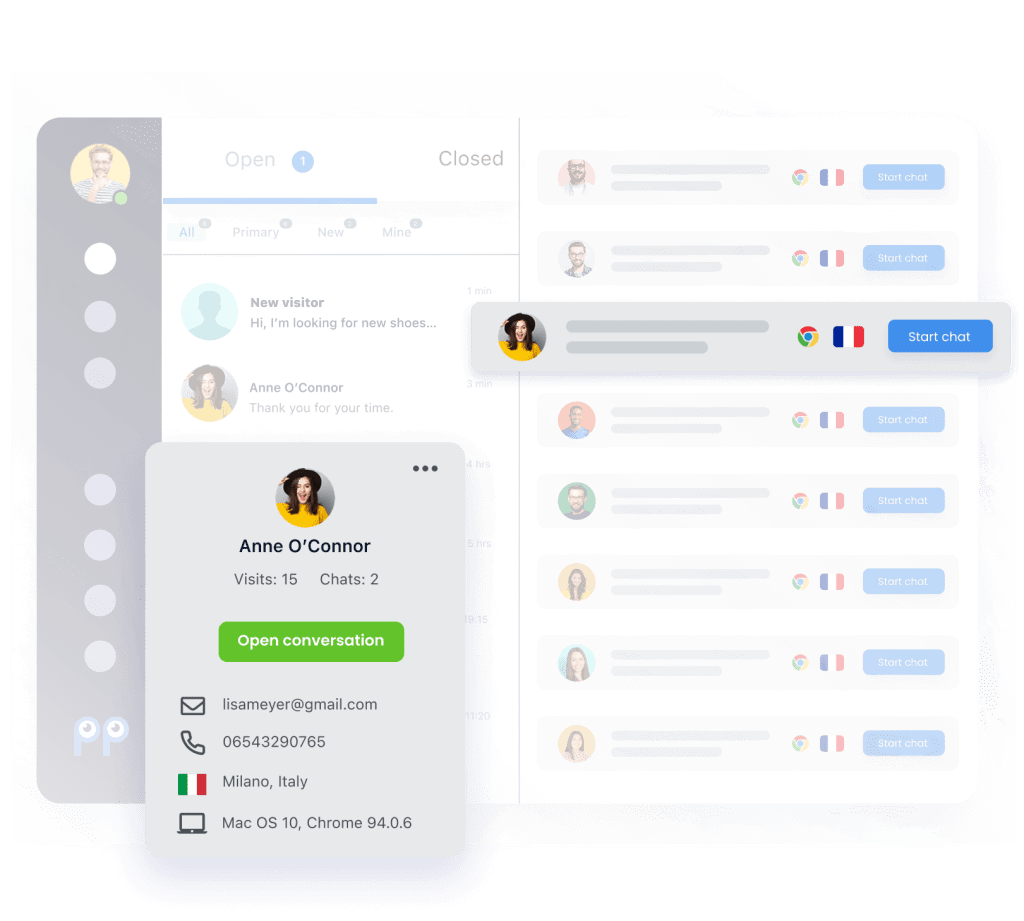
Deep PrestaShop integration
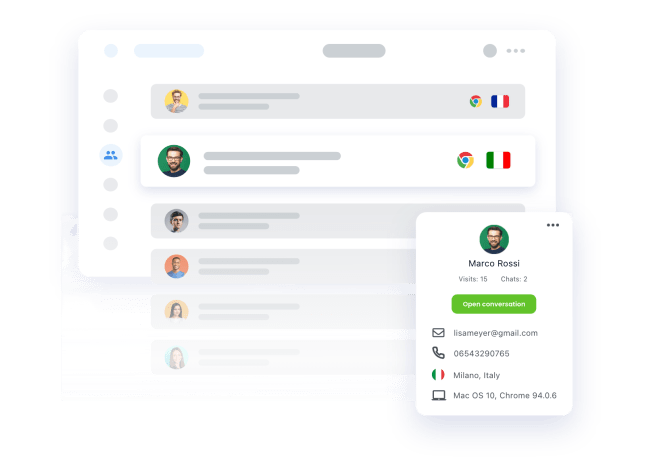
Personal details
Our PrestaShop chat plugin allows you to see your customer’s important personal details within Smartsupp, so that you can give your support the right personal touch.
Order details
See customer order history so that you can upsell them products they want and help them find better options. Smartsupp seamlessly integrates with PrestaShop, so that you can see order details and history in one place.
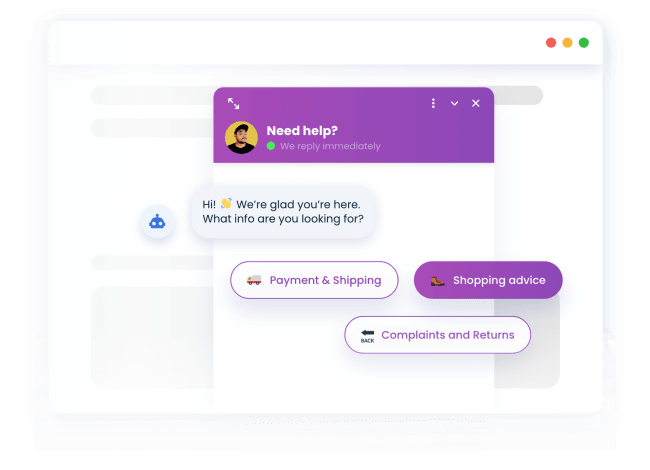
PrestaShop chatbots that sell
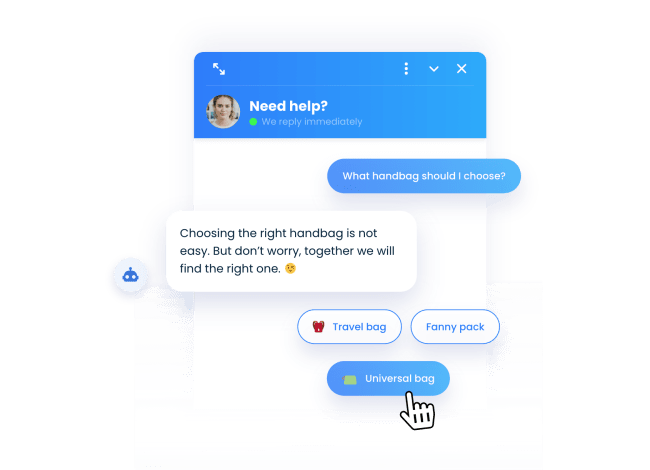
Their personal shopping guide
Our PrestaShop chatbot helps your visitors find the products they are looking for quickly and hassle-free.
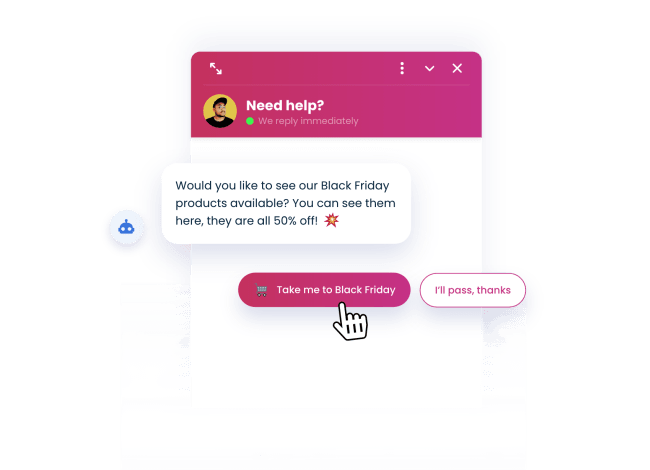
Promote your special offers
Use our chatbots on PrestaShop to promote Black Friday sales and limited time offers.
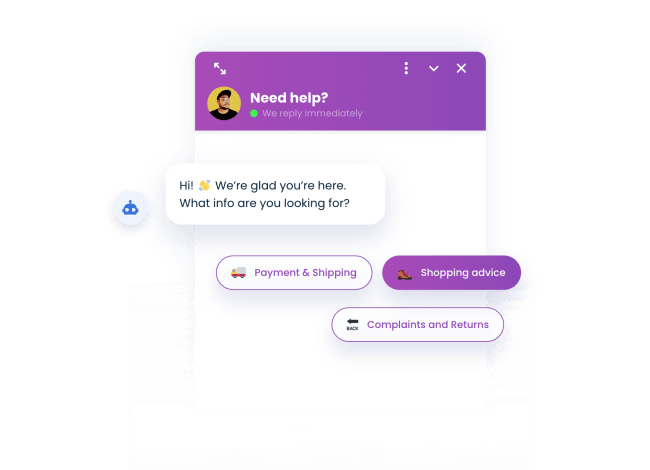
Automatically reply to FAQs
50% of the inquiries our users get are FAQs. With the FAQ chatbot for PrestaShop, you can answer them automatically.
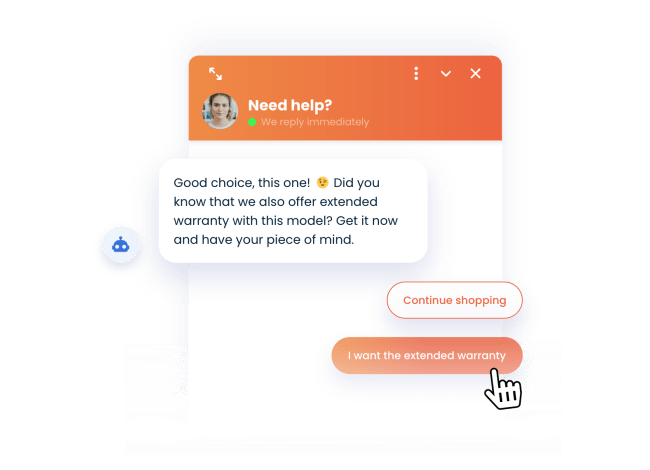
Upsell like a pro
Recommend your customers complementary products or related offers with better margins.
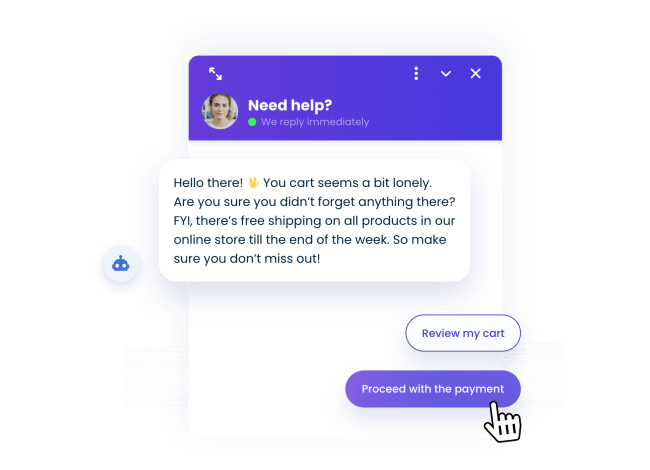
Reduce cart abandonment
Don’t let 60% of your customers abandon great products. Offer special discounts or highlight free shipping with chatbots for PrestaShop.
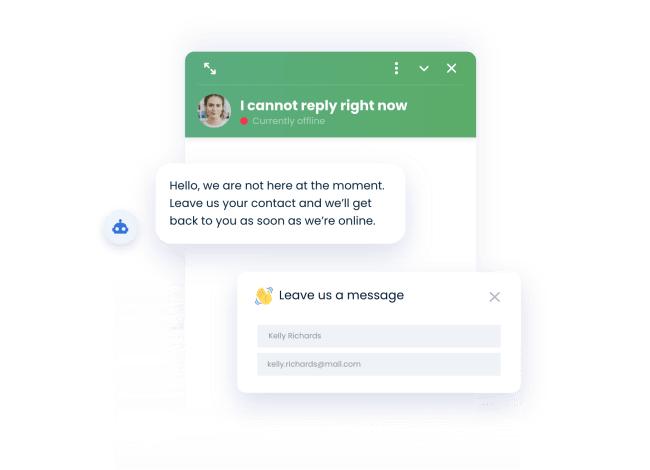
Generate quality leads
Collect email and personal details right in the chat window.
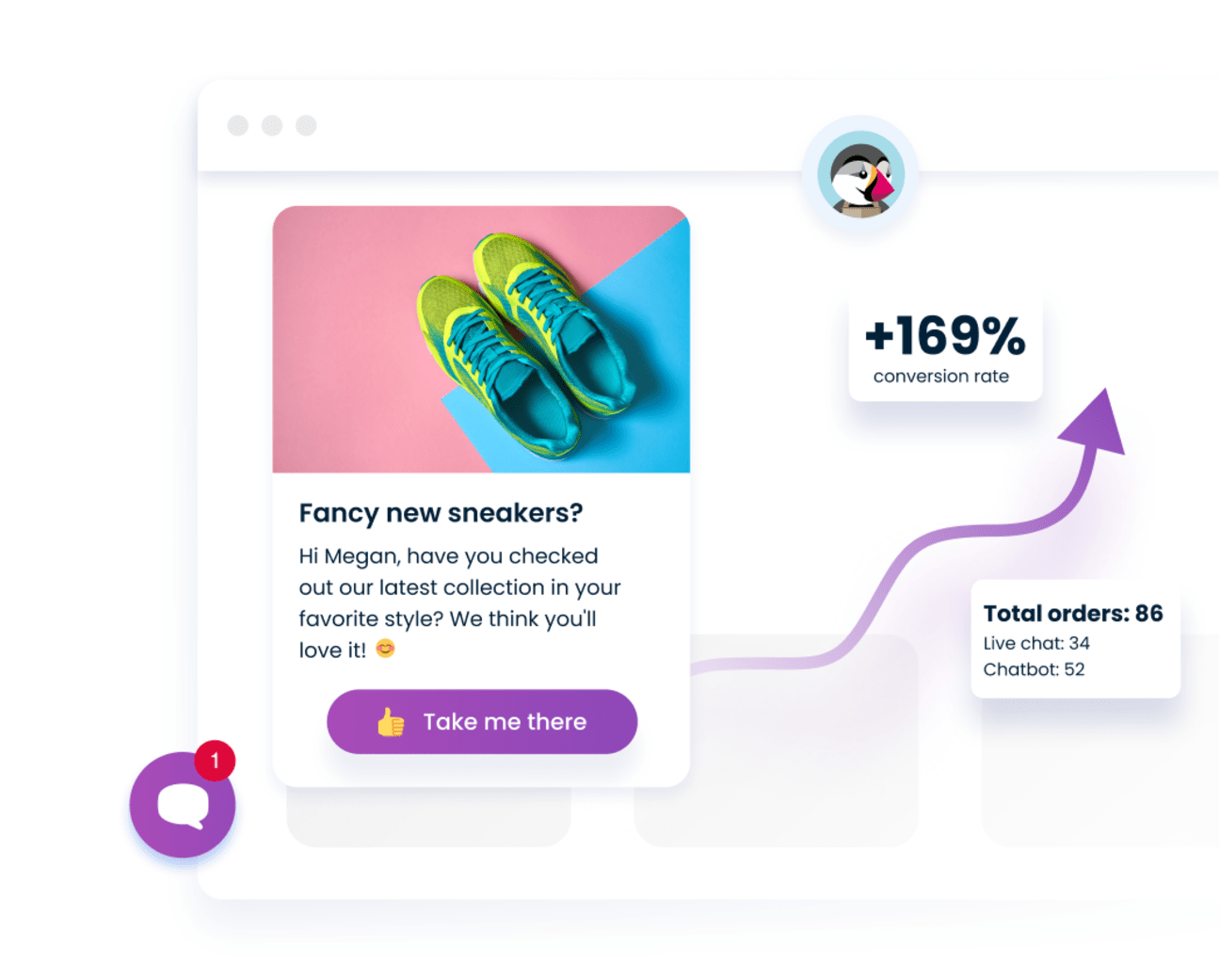
Smartsupp is proven to boost sales

“Smartsupp Live Chat helped us boost our sales by 10.8%. We've been using Smartsupp for several years and it helped us deliver a 30% higher order value, and a 169% higher conversion rate.”
Jakub Klaus
E-commerce Director of Breno
- + 169% conversion rate
- + 30% average order value
- 98% satisfaction rating
The best choice for PrestaShop!